CLEARLine® is synonymous of purity, clean and safety:
 CryoGen® Tubes CLEARLine® are certified completely free of human DNA, PCR inhibitors, DNase, RNase, Pyrogens, ATP by our internal laboratory. To download lot-specific certificate see the section certificate on the web site.
CryoGen® Tubes CLEARLine® are certified completely free of human DNA, PCR inhibitors, DNase, RNase, Pyrogens, ATP by our internal laboratory. To download lot-specific certificate see the section certificate on the web site.
 CryoGen® Tubes CLEARLine® certified as STERILE were treated with a validated method to provide a Sterility Assurance Level (SAL) of 10-6. To download lot-specific certificate see the section certificate on the web site.
CryoGen® Tubes CLEARLine® certified as STERILE were treated with a validated method to provide a Sterility Assurance Level (SAL) of 10-6. To download lot-specific certificate see the section certificate on the web site.
 Medical grade raw materials:
Medical grade raw materials:
CryoGen® Tubes CLEARLine® are produced from medical grade raw materials that will not discolor after re-sterilizing.
Medical grade raw materials are certified USP Class VI, in accordance with United States Pharmacopeia and ISO 10993.
Non Cytotoxic - Non Hemolytic Cryotubes - Heavy metals free - Ingredients of animal origin free
• The material has passed the United States Pharmacopeia testing including Class VI tests
• Material has successfully passed the biological tests according to ISO 10993 external communicating devices for indirect blood contact for a prolonged period
Test tube in medical polypropylene and cap in medical polyethylene/rubber.
The combination of polyethylene stopper and test tube in polypropylene, makes screwing easier avoiding the grip effect caused by the use of the same raw material for both components.
Barcode 128:
 Standard code 128 barcode printed on tubes’ body:
Standard code 128 barcode printed on tubes’ body:
- serial-consecutive
- unique
- human readable characters printed in both left and right hand orientation for easy readability by all users.
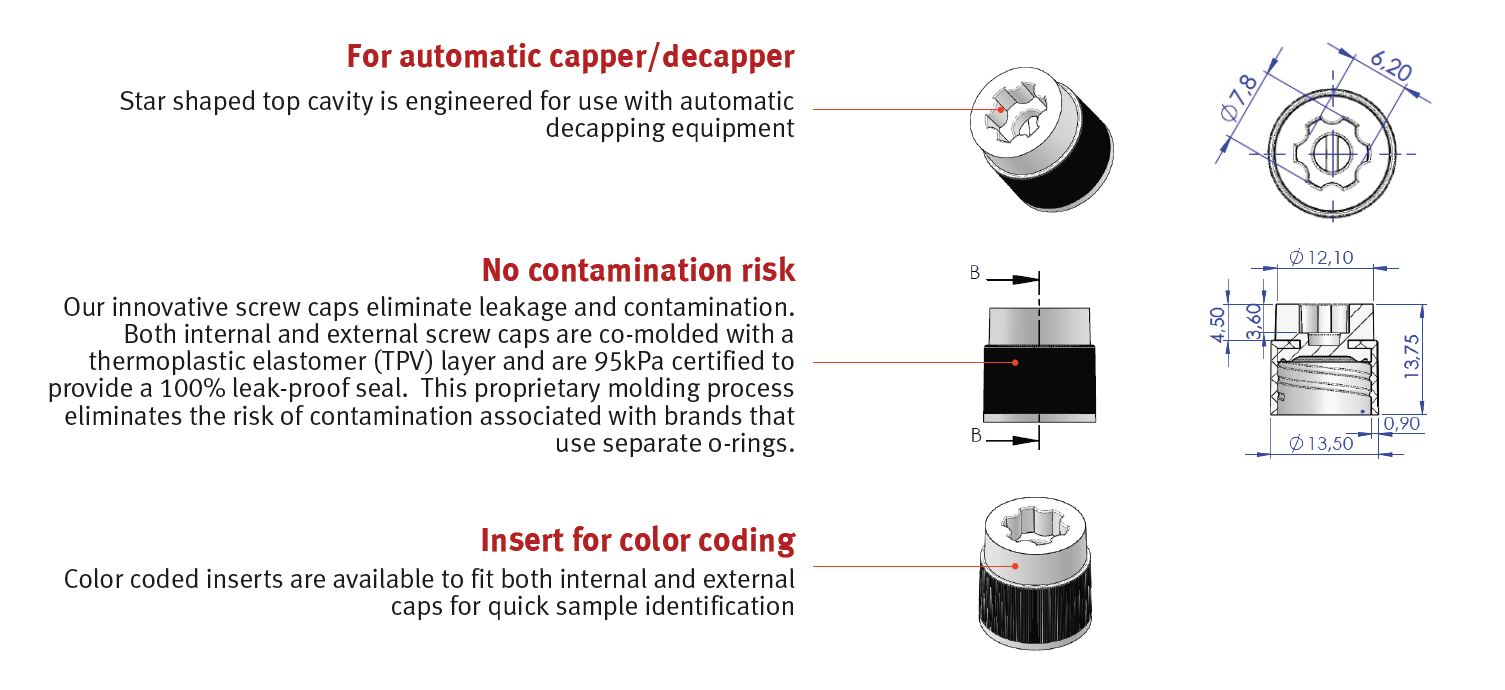
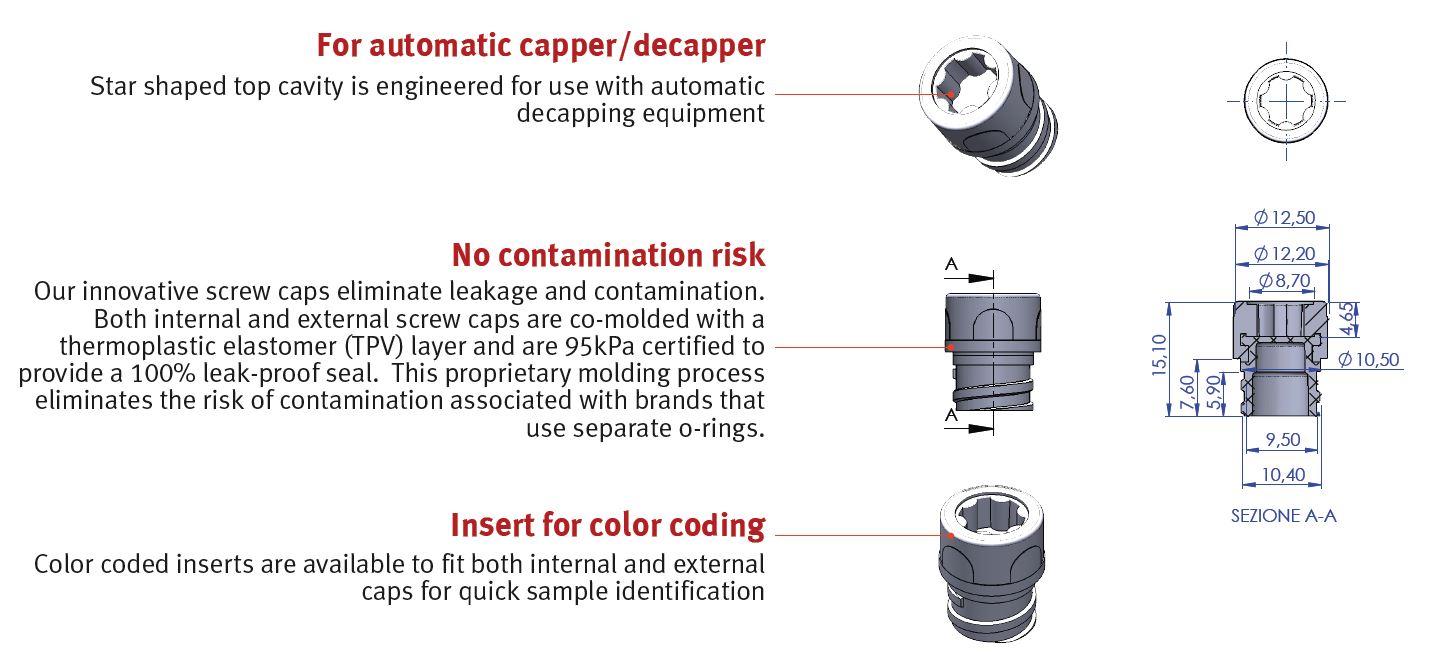
Bi-injected screw cap:
Our innovative screw caps eliminate leakage and contamination. Both internal and external screw caps are co-molded with a
thermoplastic elastomer (TPV) layer and are 95kPa certified to provide a 100% leak-proof seal.
This proprietary molding process eliminates the risk of contamination associated with brands that use separate o-rings.
Star Shape Screw cap:
Star shaped top cavity is engineered for:
- Use with automatic decapping equipment
- Use with Vial picker
- Use with Color coded inserts for quick sample identification

 Graduation and writing surface:
Graduation and writing surface:
Clear printed graduation for accurate measurement
Wide white writing surface for specimen identification
 mod (Custom).jpg) Round or self-standing bottom:
Round or self-standing bottom:
Self-standing vials interlock in workstations. Locking base features prevents tubes rotating within the rack/workstation during capping and decapping.
 Tests performed on CryoGen® Tubes CLEARLine®:
Tests performed on CryoGen® Tubes CLEARLine®:
- Test report IATA PI650
CryoGen® Tubes CLEARLine® tubes can be used as primary container for biological samples transport in conformity with packaging Instruction PI650 of the IATA regulations.
- Extractable study on CryoGen® Tubes
The extractable study performed demonstrates that the analysed vials does nor release significant amount of inorganic elements and/or organic substances in ethanol.
- Mutagenicity test – Ames test
Under the experimental conditions CryoGen® Tubes CLEARLine® CLEARLine® didn’t showed any genetic mutations on the particular bacterial strain used.
- Validation of packaging system
The integrity of the product and the packaging is resulted compliant after an accelerated aging of 5 years.
Quality controls on CryoGen® Tubes CLEARLine®:
During the production phase, visual checks and mechanical tests are performed to ensure that there are no defects (scarcity, streaks, cracks) such as:
- Check that there are no holes, deformation or burrs on both cap and tube
- Serigraphy resistance
- Verification of the correct reading of the bar code
- Verification that the matrix corresponds to the bar code for the 2D CryoGen® Tubes
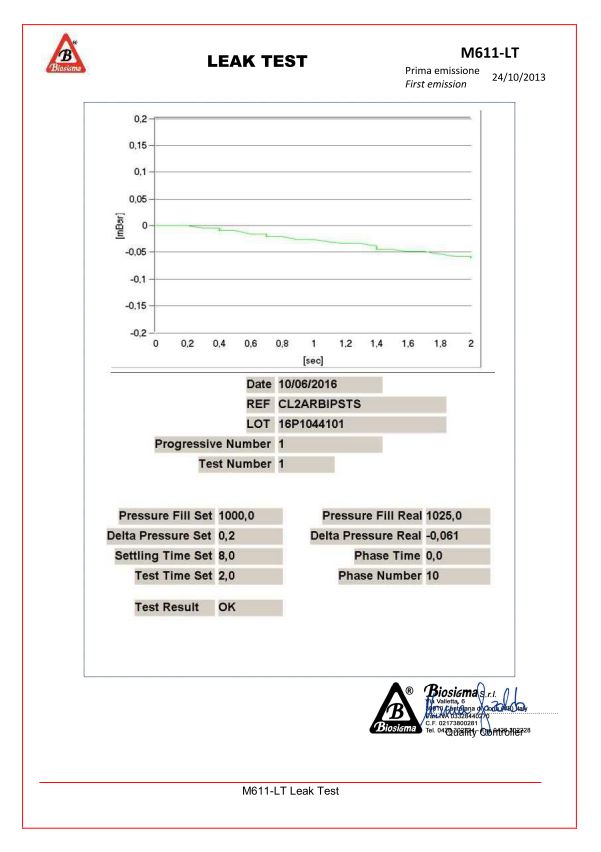
Leak test, performed with the FORTEST ET99M-VOL to verify that the pressure charge is less than 0,2 mbar, as set.
 CE-IVD marked according to the directive on in vitro diagnostic medical devices 98/79/EC - other devices.
CE-IVD marked according to the directive on in vitro diagnostic medical devices 98/79/EC - other devices.
